
Maize is one of the most produced cereal crops worldwide, with annual production levels of more than one billion tons. On average, local farmers have produced 12,5 million tons on 2,5 million ha of land over the past ten years. The production of maize is hampered by a variety of pests and diseases, which is a significant problem for both commercial and subsistence farmers in South Africa.
Pests and diseases that impact maize production include fungal, bacterial and viral diseases, insects, as well as parasitic plants and weeds, which can affect all parts of the plant. Fungal diseases in maize usually become a major problem when the ideal temperature and moisture conditions occur in the field. Commercially important fungal diseases of maize include grey leaf spot (GLS), Fusarium ear rot and northern leaf blight (NLB).
Importance of NLB
Northern leaf blight (NLB) or white rust is probably the most widespread foliar disease occurring on maize in South Africa. Typical yield losses range between 15% and 30%, but losses of up to 75% have been reported. The biggest yield losses occur when infection happens before silking and spreads to the upper leaves during grain filling. Yield losses can be attributed to the loss of the photosynthetic leaf area – in other words, the living part of the leave.
The disease happens when the fungal pathogen Exserohilum turcicum infects maize leaves. Other host plant species for this pathogen include sorghum and its related species, which is why the correct name for the disease is northern leaf blight and not northern corn leaf blight – since it can cause disease in more than one species.
NLB symptoms
- This disease is characterised by small water-soaked spots, which initially appear on the lower leaves after infection, whereafter they enlarge and form chlorotic spots (Figure 1A).
- The lesions (spots) then elongate, becoming cigar-shaped and are tan to grey-green in colour (Figure 1B). As the disease progresses, the lesions start to mature and become tan with distinct dark zones of sporulation. The lesions are usually between 2,5 cm to 15 cm long.
- Multiple lesions can coalesce to form large irregular areas of dead tissue and thus blighting the leaves (Figure 1C).
The causal agent
Exserohilum turcicum is a hemibiotrophic fungus, where the fungus initially lives as a biotroph off the living plant tissue (not killing the plant), whereafter it switches to a necrotrophic stage, killing the infected cells and completing its lifecycle (killing the plant).
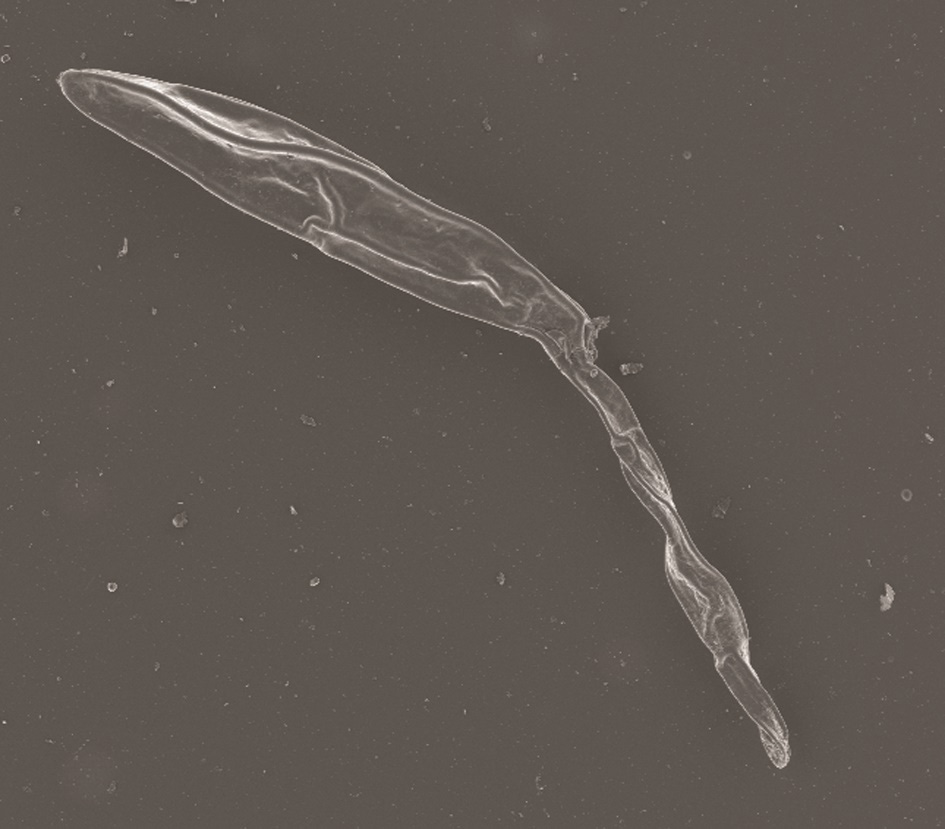
The hyphae of fungus are repeatedly branched, brown or grey to black and are 3 µm to 12 µm in diameter (Figure 2). The conidia or spores by which the fungi asexually replicates are between
73 µm and 137 µm in length and 18 µm to 23 µm in width, olive-brown in colour and slightly curved with a protruding hilum (Figure 2).
DISEASE CYCLE OF E. TURCICUM
Exserohilum turcicum survives through the winter as mycelia (accumulation of hyphae) and conidia in and on leaf debris as well as in the soil. As the temperature starts to warm up during the early spring and the moisture increases, new conidia are produced on the old residue.
Primary infection occurs when conidia spread to the lower leaves of young maize plants by wind or rain, with secondary spread occurring within fields arising from conidia formed abundantly on infected leaves. The fungus yet again overwinters in leaf debris and in the soil, thus completing its lifecycle. Conidia are the primary inoculum by which E. turcicum spreads from one leaf or plant to another and causes further infection in a field.
DISEASE DEVELOPMENT
Exserohilum turcicum infection mainly occurs during cool, wet seasons. Disease development is favoured by heavy dew, frequent rain, high humidity (>90%), moderate temperatures (20°C to 27°C), prolonged leaf wetness and photoperiod. Therefore, symptoms are usually observed after periods of heavy dew, rain and overcast days during the growing season.
Growth room studies indicated that NLB lesion progression was faster during the night than during the day. Conidial germination and appressorium formation on maize leaves were favoured by prolonging dew conditions as well as short photoperiods and low light conditions. Warm nights and long periods of high relative humidity are essential for the fungus to sporulate and complete its lifecycle.
Disease progression is usually retarded by hot and dry weather conditions and thus the disease is more prevalent in the wetter areas of the world where maize is grown.
Infection strategy
The infection process by the fungus starts when conidia on the maize leaf surface germinates and forms a germination tube which grows towards the leaf’s surface, where appressorium (penetrating structure) forms on the surface. Then the hyphae penetrate the leaf epidermal cells (leaf surface). Penetration is usually through the epidermal cell wall and rarely through the stomata. At this stage, a chlorotic fleck/spot is visually present on the leaf. This occurs one to four days after infection (Figure 1A).
As the disease progresses, intracellular growth is limited until the hyphae invade the vascular bundles (veins) and subsequently penetrate the xylem vessels and tracheids (Figure 3A). This usually occurs eleven days after the fungus has infected the leaf and it is at this stage that the lesions became necrotic and enlarge (Figure 1B).
After penetration of the vascular bundle, growth in the spongy mesophyll cells ceases. Growth throughout the vascular bundles is rapid, as opposed to the initial dilatory growth of the hyphae in the spongy mesophyll cells. Once the fungus is in the xylem, it spreads rapidly and is difficult to control. Wilted symptoms and necrosis of the bundle sheath and chlorenchyma cells follow extensive plugging of the xylem (Figure 3B).
By 14 days post-inoculation (dpi), necrotic lesions on the maize leaves had started to enlarge and elongate to form the distinct cigar-shaped lesions that are characteristic of NLB infection in maize leaves (Figure 1C). The vascular bundles are almost completely filled with fungal growth and most of the cells in the lesion have collapsed.
Between day 14 and 18 after inoculation, the fungus will accumulate in the sub-stomatal cavity and form conidia-forming structures through the stomata and thus forming new spores which can infect newer upper leaves in the plant (Figure 3B).
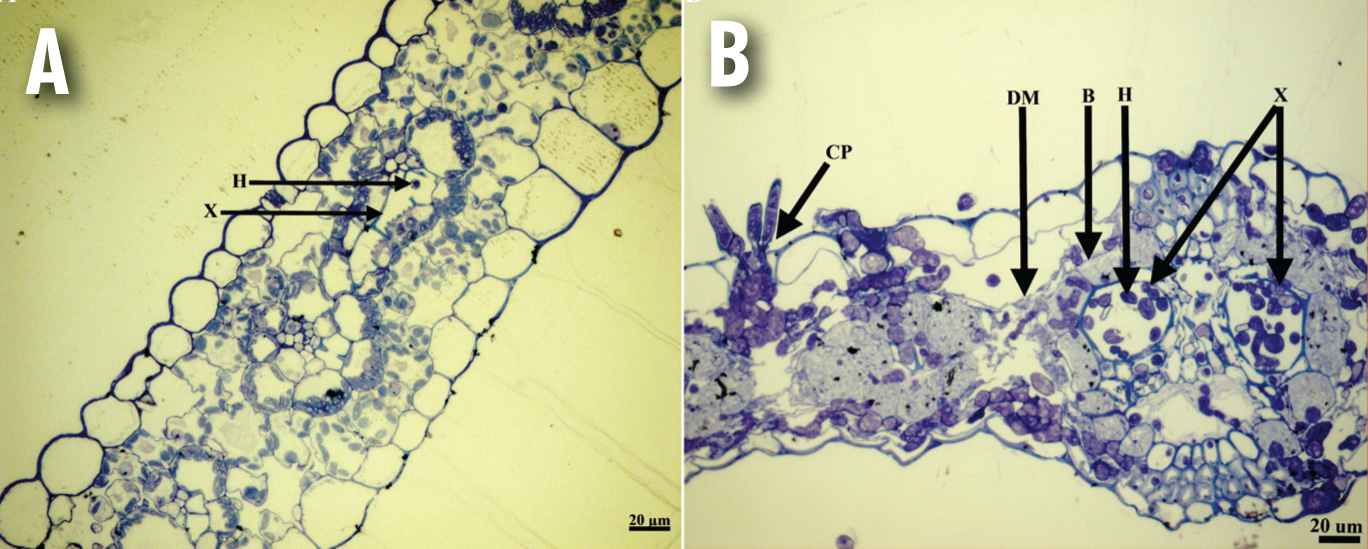
(A) The fungus present in one of the smaller vascular bundles in a maize leaf, 11 dpi;
(B) Cross section of a diseased maize leaf, 14 – 18 dpi. Complete tissue collapse can be seen with the xylem of fungal growth, with the fungus accumulating in the substomatal cavity from where the conidiophores are formed. B, bundle sheath cells; H, hypha; E, epidermal cell; X, xylem; M, spongy mesophyll cells; CP, conidiophores; DM, disintegrated spongy mesophyll cells.
Management of NLB in maize
- The management of the disease can be achieved by using deep tillage and crop rotation practices, biological control, fungicides and resistant cultivars. The use of deep ploughing or tilling practices will reduce the amount of primary inoculum carried over to the next season, since plant residue is allowed to decompose.
- Crop rotation with a non-host species of E. turcicum, such as sunflower or soya beans, will also reduce the inoculum load. In maize fields with a history of NLB and where no or reduced tillage is practised, a two-year rotation with a non-host crop should be implemented.
- Foliar fungicides are widely used by commercial farmers throughout the world to control NLB incidence in maize fields. Strobilurins and triazoles are the two major classes of agricultural fungicides currently being used to prevent NLB. The timing of fungicide applications by the farmer is extremely important, as it is easier to prevent the disease than to try and control it once it has caused a severe infection in a field. These chemical classes also run the risk of developing pathogen resistance and thus the fungicides cannot be used sustainably on their own over a long period.
Conclusion
Northern leaf blight can be severe during cool and rainy periods, which are usually observed from the end of January or beginning of February, when the day length becomes shorter and cooler temperatures prevail. It is important that producers scout their fields regularly (weekly) for the disease, especially when the plants are flowering and during grain filling. Regular scouting for the disease will lead to timely fungicide applications, preventing substantial grain losses.
References
- Hilu HM and Hooker AL, 1965. Localised infection by Helminthosporium turcicum on corn leaves. Phytopathology 55, 189-92
- Jennings P and Ullstrup A, 1957. A histological study of 3 Helminthosporium leaf blights of corn. Phytopathology 47, 707-14
- Klopper R and Tweer S, 2009. Northern corn leaf blight fact sheet. http://www.pannar.com/assets/disease_fact_sheets/Northern_Corn_Leaf_Blight.pdf. [11 November 2019]
- Kotze RG, Van der Merwe CF, Crampton BG and Kritzinger Q, 2019. A histological assessment of the infection strategy of Exserohilum turcicum in maize. Plant Pathology 68, 504-12
- Kotze RG, 2020. The host response of maize towards Exserohilum turcicum and its toxin, monocerin. Pretoria, University of Pretoria. PhD Thesis
- Levy Y and Cohen Y, 1983. Biotic and environmental factors affecting infection of sweet corn with Exserohilum turcicum. Phytopathology 73, 722-5
- Munkvold GP and White DG, 2016. Compendium of corn diseases. APS press St. Paul, MN
- Wise K, 2011. Diseases of corn: Northern corn leaf blight. https:/www.extension.purdue.edu/extmedia/BP/BP-84-W.pdf. [14 November 2019]








