According to CropLife SA, nematodes cause yield losses valued at up to R1,9 billion in Southern Africa alone. The cyst nematode causes the most damage to grain crops in South Africa. This can range from 10% to 50% in annual yield loss if not prevented or treated correctly. Biological methods of control are available that may be worthwhile for the producer to consider.
The root-knot (Meloidogyne), cyst (Heterodera and Globodera) and lesion (Pratylenchus) nematodes are major plant-parasitic pests in grain crop production. These microscopical pests cause root damage directly, which eventually appears in the leaves and stems.
Biological control strategies
FILAMENTOUS FUNGI AND BIOLOGICAL CONTROL AGENTS
Trichoderma
Trichoderma is a fungus that acts as a biological control agent (BCA) for cyst (Heterodera) nematodes in wheat crops. Trichoderma is directly added to soil, especially during planting. Trichoderma parasitises nematode J2s (second-stage juveniles) and eggs, which produces hyphae (fungal body) around nematode eggs and J2s. Zhang et al (2017) stated that the Trichoderma fungi caused deformation of the eggs and J2s, while a decrease of between 88,3% and 91,3% in juveniles and cysts was recorded in the soil.
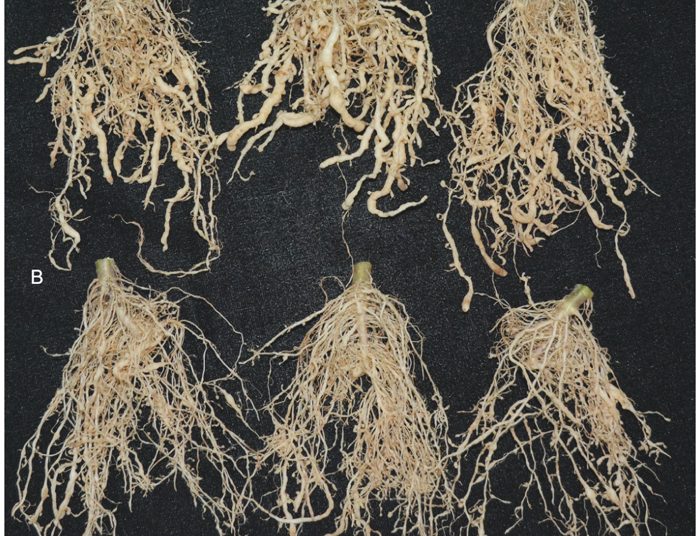
Photo 1 shows the efficiency of Trichoderma in a field experiment on tomato crops. In the top row (A), with no added Trichoderma, there is clear evidence of lesion damage caused by nematodes. The roots appear shorter and have also thickened. The roots of the plant in the bottom row (B), with added Trichoderma, had 50% less nematode infestations, which is essential for healthy root growth. Also, the Trichoderma treated plants had 13% to 17% increased shoot and root length, which may enhance the overall plant health and crop yield.
Paceilomyces lilacinus
This soil-residing fungus has proven effective control of root-knot (Meloidogyne graminicola) nematodes in rice production. Applied at 20 g/m², it showed significant results towards the control of nematode populations. The nematode population was reduced by 49% per 200cc of soil. The bio-agent also increased the root length and weight over the control treatment, which ultimately improved the yield as well.
Eucalyptus globulus
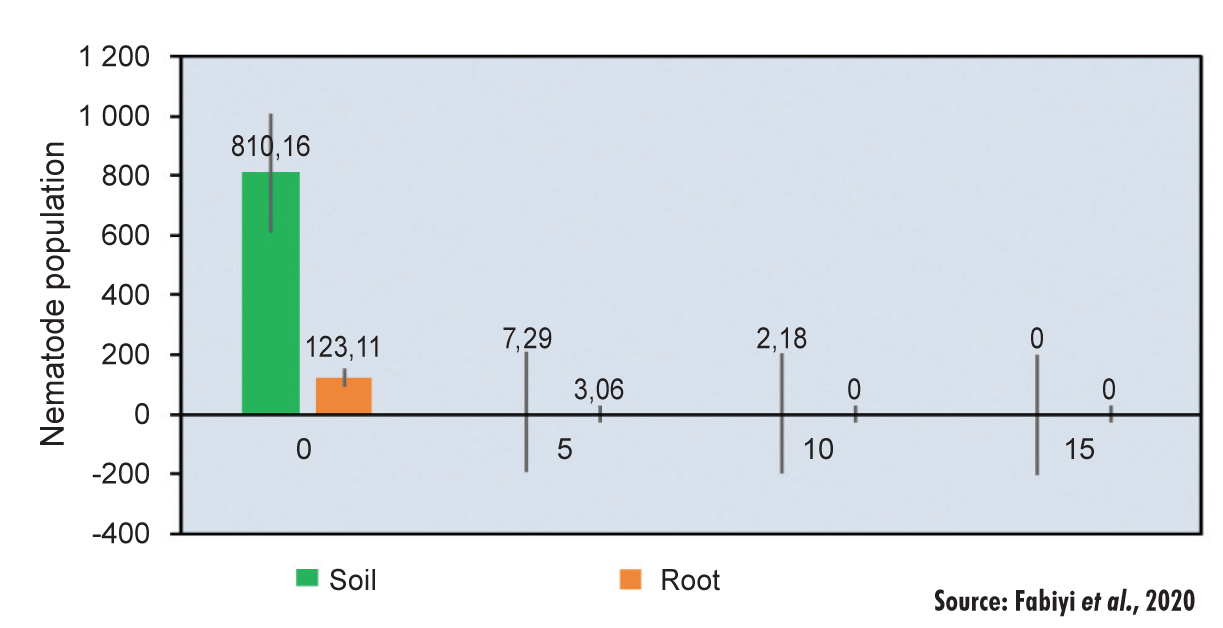
Pratylenchus nematodes are the most common in maize and may cause roots to become swollen, stunted and red/brown in colour, according to Poudyal (2007). A study was done on Pratylenchus nematodes, using Eucalyptus globulus (evergreen tree) as a biological treatment. The Eucalyptus globulus was applied to the soil at rates of 5 mg/kg, 10 mg/kg and 15 mg/kg. The bio-nematocidal plant (E. globulus) contains phytochemicals that are toxic to Pratylenchus zea eggs and juveniles. See Figure 1.
COVER AND ROTATION CROPS
Sorghum and brown top millet
Using grain sorghum, sorghum-Sudan grass and brown top millet as cover or crop rotation, can prevent the reproduction of Meloidogyne enterolobii (root-knot) nematodes. Roots of several crops were inoculated with great amounts of nematodes to test the effect of sorghum and brown top millet on nematode reproduction rates.
Although these crops were inoculated with large amounts of root-knot nematodes, the reproduction of eggs was not visible. Only some J2 and J3 were present, which did not reach adulthood. Sorghum and millet are excellent to use as summer cover crops to reduce root-knot nematode populations, and also to serve as a sustainable and environmentally friendly control strategy.
Canola
Brassicas such as canola contain glucosinolate, which are secondary metabolites. Such compound can create isothiocyanate and nitrile gasses, which have toxic effects on nematodes. It has been found that canola and several other Brassicas prevent nematodes from completing a lifecycle, which automatically leads to reduced nematode reproduction and populations.
Pigeon pea and Sunn hemp
Both crops can suppress nematode activity and reduce populations. Crops from the Fabaceae (legumes) family produce nematotoxicity compounds, which may prevent J2s from developing into females.
Summary
- The use of BCAs and cover/crop rotations may suppress and even eliminate nematodes of the Meloidogyne, Heterodera and Pratylenchus genus in crop production.
- BCAs are funguses that are directly applied to the soil. The funguses stick and grow onto nematode bodies, which prevents nematodes from functioning or reproducing.
- Many BCAs also enhance plant growth parameters, such as root length, root mass and overall plant health. Enhanced growth parameters may also increase the plant’s ability to be more resistant or tolerant to nematode activity.
- Cover/crop rotation is used worldwide to biologically suppress nematodes. The main goal of such systems is to provide non-hosts for nematodes, which automatically reduces the nematode population for the next growing season.
References
- Amaral DR, Oliveira DF, Campos VP and Carvalho DA, 2002. Efeito de alguns extratos vegetais na eclosão, mobilidade, mortalidade e patogenicidade de Meloidogyne exigua do cafeeiro. Nematologia Brasileira 26, 43-48
- Fabiyi OA, Atolani O and Olatunji GA, 2020. Toxicity effect of Eucalyptus globulus on Pratylenchus spp. of Zea mays. Sarhad Journal of Agriculture, 36(4). http://dx.doi.org/10.17582/journal.sja/2020/36.4.1244.1253
- Fan H, Yao M, Wang H, Zhao D, Zhu X, Wang Y, Liu X, Duan Y and Chen L, 2020. Isolation and effect of Trichoderma citrinoviride Snef1910 for the biological control of root-knot nematode, Meloidogyne incognita. BMC microbiology, 20, pp.1-11
- Ferreira PS, Torres JLR, Dos Santos MA, De Oliveira Parolini R and Lemes EM, 2020. Host suitability of cover crops for Meloidogyne javanica and M. incognita. Nematology, 22(6), pp.659-666
- Khanal C and Harshman D, 2022. Evaluation of summer cover crops for host suitability of Meloidogyne enterolobii. Crop Protection, 151, p.105821. https://doi.org/10.1016/j.cropro.2021.105821
- Narasimhamurthy HB, Ravindra H, Sehgal M, Ekabote SD and Ganapathi G, 2017. Bio-management of rice root-knot nematode (Meloidogyne graminicola). J Entomol Zool Stud, 5(4), pp.1433-1439
- Neves DL, Ribeiro LM, Dias Arieira DR, Campos HD and Ribeiro GC, 2012. Sobrevivência de Pratylenchus brachyurus em diferentes substratos, com baixo teor de umidade. Nematropica 42, 211-217
- Poudyal DS, 2007. Plant-parasitic nematodes associated with maize in Chitwan. Acoustics, Speech and Signal Processing Newsletter. pp. 25. https://doi.org/10.3126/jiaas.v25i0.392
- TariqJaveed M, Farooq T, Al-Hazmi AS, Hussain MD and Rehman AU, 2021. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. Journal of Invertebrate Pathology, 183, p.107626. https://doi.org/10.1016/j.jip.2021.107626
- Zhang S, Gan Y, Ji W, Xu B, Hou B and Liu J, 2017. Mechanisms and characterisation of Trichoderma longibrachiatum T6 in suppressing nematodes (Heterodera avenae) in wheat. Frontiers in plant science, 8, p.1491. https://doi.org/10.3389/fpls.2017.01491