
It has become common practice to use large quantities of reduced nitrogen (urea, ammonia and ammonium), especially urea, as a preplant fertiliser, when producing maize. This article looks at the risk of ammonium toxicity, explains its effects and suggests how it can be prevented or alleviated.
The practice of using large quantities of reduced nitrogen as a pre-plant fertiliser for maize production is well established in the Free State and North West, and it is also finding its way into Mpumalanga. These products pose the risk of a build-up of the nitrogen cation, ammonium, which is highly toxic to plants.
When large amounts of urea, for instance, are pre-planted at a depth of 200 mm to 300 mm in typical sandy soil in the North West and Free State areas, it is usually in a fairly cold, acid environment, which is low in organic matter. During the early season, after the first rains, such soil often becomes waterlogged. These conditions are conducive to the accumulation of ammonium, as the process of nitrification is inhibited.
Ammonium toxicity
Ammonium toxicity was described by Charles Darwin in 1882. A plant cannot store ammonium in the same way as it does with nitrates. Free ammonium in the plant cell is toxic and needs to be bound in amino acid form by combining it with sugars as soon as possible.
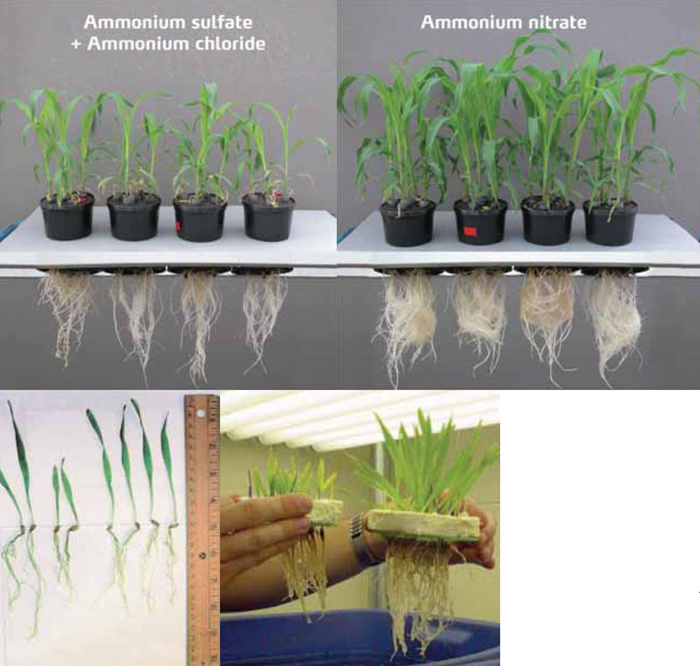
If it is the only nitrogen source, it already has a toxic effect on grain crops at levels as low as 150 mg/kg in the soil. This level is already reached when 80 kg nitrogen is banded in one-metre rows in sandy soil. It causes severe plant chlorosis – often similar to a magnesium deficiency symptom, which is yellow striped leaves – with reduced photosynthesis, stunted plants with a low shoot to root ratio and inhibition of especially root growth (see photos).
Ammonium toxicity can have a severe impact on seedling growth in particular, but also on mature plants, and can lead to between 15% and 60% yield loss. Ammonium toxicity is so aggressive that it can even be used as a herbicide.
Some physiological characteristics related to ammonium toxicity are:
- The suppression of cation uptake (calcium, magnesium and potassium).
- The enhancement of anion uptake (especially phosphate and chloride).
- Other indicators are a reduction of intra-cellular pH and a drastic decline in sugars and starches in the cell.
Studies on maize in South Africa by the Agricultural Research Council (ARC) has indeed proven such toxicity, showing severe yield depression in maize at high nitrogen applications with urea as product.
Nitrification and acidification
The oxidation of ammonia is dependent on several factors. Nitrification is dependent on the presence of nitrifying bacteria, which are sensitive to low temperatures, the pH and basic cations such as calcium.
In fact, it is documented that nitrification comes to a complete halt at pH levels (measured in water) of 5,5. Also, nitrification is severely inhibited by a lack of oxygen and ironically high levels of ammonium. In sandy, waterlogged subsoils with a low pH and temperature, typically associated with the soils of the Free State and North West, 50% nitrification of ammonium may take as long as 50 days. Nitrite toxicity is also often associated with delayed nitrification.
It is well known that, if a reduced nitrogen source is not efficiently intercepted by the root system, it will convert to nitric acid, causing severe soil acidification. This would then also be expected if high concentrations of urea and ammonium are used, especially when pre-planted.
Subsoil analysis trends
An analysis of the data from 5 200 subsoil samples from the Omnia soil analysis database, since 2002 in the areas of Bothaville, Viljoenskroon and Hoopstad, has confirmed the above-mentioned theory and revealed the following disturbing facts:
- A drop in the median pH (KCl) of more than 0,4 units to a critical level of 4,7 (40% of all the samples had a pH of less than 4,5, with the lowest measured being 3,5).
- A significant increase of extractable soil acidity from zero to more than 10% for more than one in five of the samples in 2011 was measured (the highest being 53%).
What is also of note, is the lack of presence of cations in the subsoil. Half the samples had calcium, magnesium and potassium levels of less than 330 mg/kg, 60 mg/kg and 70 mg/kg respectively.
The remedy
The following question may be asked: What can be done to remedy the situation of the subsoil ammonium toxicity threat, especially as reduced nitrogen sources will remain popular due to its high concentration and availability?
- Suggestion number 1 is to get cations into the acidified subsoil zone by means of fertilisers containing a high cation concentration and by using combinations of lime and gypsum. It has been proven that higher cation presence (especially calcium), even under low pH conditions, will enhance nitrification significantly.
- Suggestion number 2 is to simply add a fraction of nitrate to the reduced fertiliser source. Literature states that the nitrogen uptake may be enhanced by 75% and the growth rates of crops may be enhanced by up to 40% and even higher, using combinations of reduced nitrogen and nitrate. The ratios between ammonium and nitrate are specific to the crop produced, but in general for grains a ratio of 80% reduced nitrogen and 20% nitrate nitrogen is near the optimum.
Some physiological explanations of this enhancement of reduced nitrogen efficiency with nitrates is the stimulation or optimisation of biochemical processes, stimulation of the synthesis of the growth hormone cytokinin, physiological alkalisation of the rhizosphere (hydroxyl swop from the root for negatively charged nitrate) and preventing carbon drain from the root.
Speak to your Omnia agronomist to get the right tailormade product to manage the risk of ammonium toxicity in your crop, especially for pre-planting and with planting.
Sources
- Adriaanse FG, 2012. Efficacy and application of nitrogen sources. Proceedings of the Technical Symposium of the Fertiliser Society of South Africa, Pretoria, 22 August 2012. Pages 4 – 19
- Britto DT and Kronzucker HJ, 2002. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 159. Pages 567 – 584








